Introduction
We are going to study the model of an OxyFuel type oxycombustion cycle.
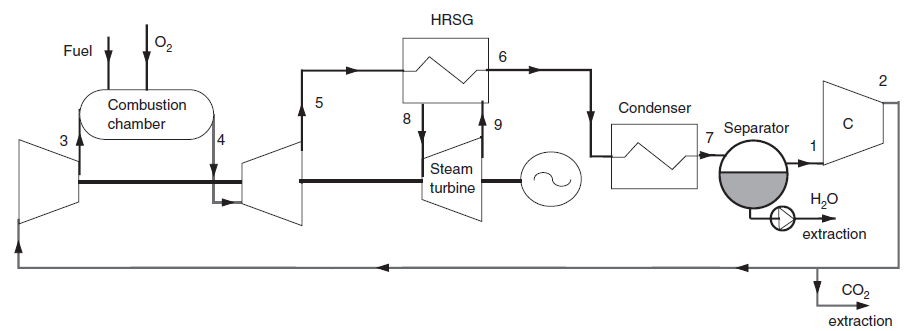
Carrying out an oxycombustion consists in replacing the usual oxidizer, namely air, mixture mainly of oxygen O2 and nitrogen (resp. 21% and 78% by volume) by pure oxygen or a mixture of oxygen O2 and of carbon dioxide CO2CO2. Oxy-combustion techniques allow one both to obtain fumes composed almost exclusively of water and carbon dioxide and to greatly reduce nitrogen oxide emissions.
 oxyFuel sketch
oxyFuel sketch
In an OxyFuel type oxycombustion, cycle, at the condenser inlet, we get fumes containing CO2 and H2O at a pressure of 1 bar. They are cooled by an external heat sink at a temperature low enough for almost all water to be condensed (7). The condensed water is extracted from the cycle.
At the condenser outlet, a fraction of the gas composed mainly of CO2 is extracted (2), the remaining flow, equal to 35 kg/s in this example being compressed at the pressure of 40 bar in a compressor (2–3) before being mixed with pure oxygen (about 4.4 kg/s). This mixture is then used as an oxidizer in a stoichiometric combustion chamber (3–4) whose fuel is methane, the temperature at the end of combustion being about 1350 °C.
Fumes are expanded at 1 bar in a turbine (4–5), then cooled in a recovery steam generator (5–6) before entering the condenser, thus closing the cycle.
A steam cycle, working between pressures of 0.03 bar and 150 bar and 530 °C, involving a water flow of 10 kg/s, is coupled to the previous cycle to form a combined cycle. In the diagram of this Figure, a simplified steam cycle is represented as a HRSG and the turbine pump and condenser are not shown nor oxygen and fuel compressors.
Such a model leads to the following performances: power 35 MW, gross efficiency 60.6%, but these excellent values must be tempered by the "cost" of separating oxygen from the air.
Loading the OxyFuel oxycombustion cycle model
Let's study the OxyFuel oxycombustion cycle model.
Loading the model
Click on the following link: Open a file in ThermoptimOpen a file in Thermoptim
You can also:
- either open the "Project files/Example catalog" (CtrlE) and select model m15.2 in Chapter 15 model list.
- or directly open the diagram file (OxyFuel2_En.dia) using the "File/Open" menu in the diagram editor menu, and the project file (OxyFuel2_En.prj) using the "Project files/Load a project" menu in the simulator.
LThe setting of this model requires some explanation.
Paramétrage du modèle
This cycle, which is a somewhat special combined cycle, has three main parts:
- the gas turbine, at the top of the diagram
- the steam cycle, in the middle of the diagram
- the system for separating carbon dioxide and water, at the bottom of the diagram
The special feature of the gas turbine is that its oxidizer only contains oxygen, water and carbon dioxide. Its fuel is natural gas, and combustion is stoichiometric, so that the fumes are composed only of carbon dioxide and water, the oxygen supply being regulated for this.
The steam cycle is very close to that which has been studied in guided exploration on the single pressure (C-M3-V1).
The system for separating carbon dioxide from water uses an external class called ColdBattery, capable of simulating the cooling of a humid gas with condensation of the water it contains.
Its configuration requires specifying the efficiency of water extraction, and the temperature of the water extracted. The model calculates on the one hand the thermal power of cooling, transmitted via a thermocoupler to a cooling process, and on the other hand the composition of the cooled and dehumidified gas.
CO2 extraction is easily done using a divider, one of the branches of which has an set flow rate: the rest of the CO2 is extracted from the cycle.
Application exercises
You can now perform sensitivity analyzes of the performance of this cycle to its many parameters.
Conclusion
This exploration allowed you to discover an OxyFuel type oxycombustion cycle model and the specific settings it uses.